Australopithecus habilis!
Wed, Aug 18 2010 02:42 | Palaeoanthropology | Permalink

Stone tools found during excavations in the early 1990s in the Afar region of Ethiopia have been dated to between 2.5 and 2.6 million years. However, H. habilis does not first appear on the scene until around 2.3 million years ago, which would make australopithecines or paranthropines the most likely authors of this assemblage. Even these earliest stone tools have been deemed too advanced for our first foray into stone tool making and many researchers predicted that even earlier tools were awaiting discovery.
Researchers working in the Dikika region of Ethiopia have recently uncovered bones dating to between 3.2 and 3.4 million years ago that show all the hallmarks of butchering. The cut marks and percussion marks are suggestive of defleshing and the removal of bone marrow. From a behavioural aspect, it is unclear whether this represents hunting or the scavenging of recently dead animals.
Bone trauma can be an incredible tricky thing to interpret. Trampling, tooth marks from scavenging, direct contact with rocks, among other agents can leave pseudo-cut marks on a bone. The bones were analysed under scanning electron microscope, with the researchers concluding that stone tools were most likely responsible for the cut marks and fracture patterns.
Australopithecus afarensis is the only known hominin to date from this time period and is, for the time being, the best candidate for making these marks. Tool use is seen in both our ape and monkey cousins and it seems likely that A. afarensis also utilised tools. Researchers have shown that A. afarensis would have been capable of the manual dexterity needed to manipulate tools. What is less clear is whether these cut marks were made by stone tools specifically fashioned for butchering or whether these hominins used sharp-edged natural stones. Whether these were fabricated or natural they were still used as tools. However, the dentition of A. afarensis suggests that meat constituted a negligible part of their diet. The large molars and thick enamel of this hominin point to a diet rich in tubers and other vegetation.
The elephant in the room is the absence of any tools at the Dikka site. This is unusual since tools, which ordinarily preserve better, typically outnumber bones at butchering sites. It is also unclear how many bones were collected at the site and why none of these show tool marks. Indeed, the entire evidence consists of only two small fragments of fossilised bone. The authors suggest that the lack of additional bones with cut marks could indicate that the bones were processed off-site, where better quality tools were available.
The evidence is tantalising but more is needed. Hopefully, further excavations at Dikka will uncover the missing stone tools and the humans who made them.
References
Alba DM, Moyà-Solà S, Köhler M. 2003. Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. Journal of Human Evolution 44: 225–254.
McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Bearat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikka, Ethiopia. Nature 466:857-860.
Semaw, S., Renne, P., Harris, J.W.K., Feibel, C., Bernor, R., Fesseha, N. and Mowbray, K. 1997. 2. 5 million-year-old Stone tools from Gona, Ethiopia. Nature, 385:333-338.
View Comments
Australopithecus sediba: an evolutionary mosaic
Thu, Apr 15 2010 01:29 | Palaeoanthropology | Permalink

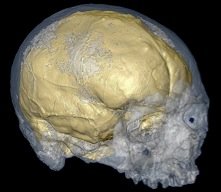
The skull of MH1, a juvenile member of the species Australopithecus sediba.
Two beautifully preserved partial skeletons of a new species of human are described in the current issue of Science magazine. The new species has been given the taxonomic name Australopithecus sediba. The remains were discovered in Malapa, South Africa, located a mere 15km from the famous Sterkfontein caves. The site preservation is incredible, especially considering its great antiquity. The specimens themselves are relatively free from distortion and show few signs of taphonomic modification.
The fossils are around 2 million years old based on a combination of radiometric and palaeomagnetic dating, as well as the associated animal remains found at the site. The skeletal remains are those of an adult female and a boy of between 9 and 13 years. A. sediba would have stood at about 1.3 metres tall and had relatively long arms like those seen in other australopithecines.
Palaeoanthropologists are split on whether these fossils are members of our genus, Homo, or the earlier Australopithecus. The boy's brain, which is estimated to be around 95% its projected adult size is only 420 cc, some 90 cc below the smallest brain known for early Homo (with a brain case of only 510 cc, KNM-ER 1813 itself is considerably smaller than other Homo specimens). It is on a par with the cranial capacity of the diminutive species Homo floresiensis.
The Malapa hominins have a mix of both australopithecine and Homo traits, with the authors of the paper suggesting greatest specific affinities to A. africanus. The small body, long arms and small brain case are indeed more suggestive of australopithecines. A. africanus, itself is a very variable species and it would not be absurd to suggest that the Malapa hominins represent one tail of the bell curve of variation within that species. The biggest difference between the Malapa hominins and A. africanus is the small dental dimensions of the former. Other traits are more typically associated with Homo, such as long legs, short hands, a derived pelvic configuration, gracile jaw with a weakly developed chin, small teeth, a flat face and a projecting nose. This mosaic anatomy should be a warning to palaeoanthropologists wishing to identify species based on a single anatomical feature.
It has been suggested that A. sediba could be a candidate ancestor for Homo, based on the number of derived traits it share with early representatives of that genus (more than any other known australopithecine). While the site is too late to be ancestral to Homo, the species may not be.
So should sediba be classified in the genus Australopithecus or Homo? The traditional way of distinguishing Australopithecus from Homo was the larger brain size of the latter (with a cutoff point of around 600 cc) and its use of stone tools. Using of a trait like brain size is highly problematic, since it is strongly correlated with body size and there is not a one-to-one correspondence between brain size and brain function. The recent discovery of H. floresiensis, with its small but derived brain, was found together with sophisticated stone tools. Similarly, a preliminary analysis of A. sediba suggests that its brain is more derived than its size would suggest. The first unambiguous appearance of stone tools in the palaeoanthropological record are attributed to H. habilis. Stone tools have not been recovered from Malapa but formal excavations have yet to get underway there. If stone tools are recovered it will require a rethinking about how we define our genus. While brain size is not the only distinguishing characteristic palaeoanthropologists use to separate Homo and Australopithecus, the dividing line is nonetheless an arbitrary one. For the moment, I think Australopithecus is a reasonable preliminary designation for this material, particularly considering our incomplete knowledge of the fossil record.
News headlines touting A. sediba as the "missing link" between humans and apes is misguided on multiple levels. The term "missing link" comes from an outmoded understanding of evolution. Moreover, humans did not suddenly appear with Homo. This is a gross over-simplification of how evolution works. We should not expect to see a momentous change between the first members of a new species or genus and their parent population. Indeed, there is considerable debate as to whether members of the species H. rudolfensis (e.g. KNM-ER 1470) and H. habilis (e.g. OH 24 a.k.a. "Twiggy"), which lie on the generic dividing line, would actually be more accurately classified as australopithecines. I've seen grown men (it seems to be men that get most bent out of shape about such technicalities) argue vehemently over such taxonomic subtleties. Evolutionary theory would dictate that the line between Homo and Australopithecines be a fuzzy one. In fact, if we had a complete fossil record it would be near impossible to know where to draw the line between different genera and species.
In the meantime, more individuals are being slowly uncovered at Malapa. Among these finds, are the arms bones of a 12 – 18 months old infant uncovered metres away from the two published specimens. Whether A. sediba maintains it australopithecine designation or not, is much less interesting than what this population tells us about hominin variation circa 2 million years ago.
References
Lee R. Berger, Darryl J. de Ruiter, Steven E. Churchill, Peter Schmid, Kristian J. Carlson, Paul H. G. M. Dirks, Job M. Kibii (2010). Australopithecus sediba: A New Species of Homo-Like Australopith from South Africa Science, 328, 195-204: 10.1126/science.1184944
Taking a walk along our evolutionary trail
Mon, Apr 5 2010 07:07 | Palaeoanthropology | Permalink
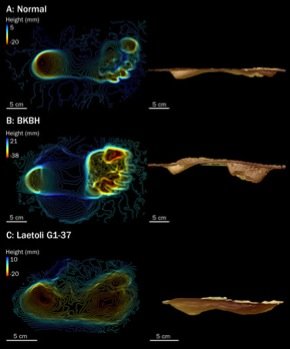
Some 3.6 million years ago the now extinct Sadiman volcano erupted in Laetoli, Tanzania. It released a plume of ash into the atmosphere. This was the rainy season and the rains changed ash into mud. Elephants, antelopes, hares, giraffes, pigs, rhinos, as well as some bird species walked over the muddied terrain. Among the footprints were those from a pair of (and perhaps even three) hominins, walking side-by-side. A second eruption released more ash into the air covering over the footprints, preserving them as a layer of tuff.
And so the it remained for more than three-and-a-half million years.
Mary Leakey sent an expedition to investigate Laetoli in 1974. One afternoon in 1976, a group of paleontologists were passing the time by throwing elephant dung at each other. Admidst the mud flinging, palaeontologist Andrew Hill found himself standing atop the now eroded ash layer. Archaeologists set about painstakingly excavating the footprints. The layer was friable and crumbled easily. After years of meticulous excavation, the footprints were exposed in all their glory; the grand prize being the fifty metre trail left by the hominins.
They are perhaps the clearest evidence for the early adoption of bipedal walking in our lineage. The footprints are thought to belong to Australopithecus afarensis, the species which included the famous fossil Lucy. However, there has been some debate as to whether these tracks represent fully bipedal locomotion or were more similar to the bent-knee, bent-hip gait seen when modern chimpanzees adopt a bipedal locomotion.
In a study that recently appeared in the journal PLoS ONE, human subjects were asked to walk over a specially constructed walkway. The surface of the track was covered with a damp sand, to mimic the soft underfoot condition that existed at Laetoli when the footprints were laid-down. The subjects walked twice across the trackway and then a further two times assuming a bent-knee, bent-hip gait. Walking with a normal modern human gait produced foot impressions with nearly equal heel and toe depths. In contrast, the bent knee gait resulted in footprints with deeper toe impressions than heel impressions. When non-human apes walk bipedally, weight is transmitted from the heel, along the outside of the foot, with toe-off occurring around the middle of the foot. We on the other transmit weight along the heel to the ball of the foot, finally toeing-off with the big toe. This is the more efficient way to walk bipedally. The impressions from Laetoli best match the pattern made by modern humans.
However I would be cautious about drawing too many conclusions from this study. One major drawback of this study is that walking with a bent-knee, bent-hip gait is not a natural gait for us. The impressions left by modern humans walking with this posture are probably not exactly the same as the footprints that a chimpanzee would leave when walking upright. While this study suggests that these hominins walked with a gait similar to our own, there is still room for debate as to exactly how similar the footprints are to our own. Regardless of these drawback, this study is a step in the right direction (no pun intended).
References
Raichlen, D., Gordon, A., Harcourt-Smith, W., Foster, A., & Haas, W. (2010). Laetoli Footprints Preserve Earliest Direct Evidence of Human-Like Bipedal Biomechanics PLoS ONE, 5 (3) DOI: 10.1371/journal.pone.0009769
The abrupt increase in brain size that wasn't?
Thu, Apr 1 2010 07:58 | Palaeoanthropology | Permalink
In an interview that recently appeared in the Guardian, neurobiologist Colin Blakemore has overstepped the mark in his discussion of the evolution of the human brain. There are a number of problems with Blakemore's thesis that have been covered more than adequately by Jerry Coyne and John Hawks. I wish to focus on the claim that there was an "abrupt" increase in brain size in hominins around 200,000 years ago. Blakemore presents his argument as follows:
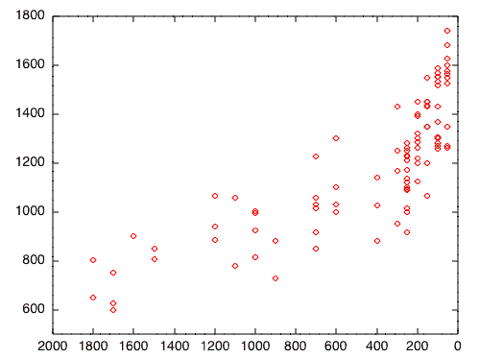
John Hawks is not convinced that there is any abrupt change in cranial capacity. Referring to the above graph showing endocranial volume against time he writes:
I decided to take the data from the Lee and Wolpoff paper and compare the periods prior and subsequent to 200,000 years ago. As Hawks eluded to, the data can be explained by a linear model. However, this is not very helpful since we can easily fit a line or curve to just about any data. More to the point, a single fitted line doesn't tell us much about any changes in the data. The red line in the graph below corresponds to the best fit line for the entire dataset (r = 0.81). The green and orange lines are the best fit lines for the two time periods we are considering. We can see that slopes of all three lines differ appreciably from one another. An analysis of covariance test confirms that there is a significant difference in cranial capacity between the two time periods, after we control for time. The model is statistically significant: F(1, 84) = 107, p < 0.001.
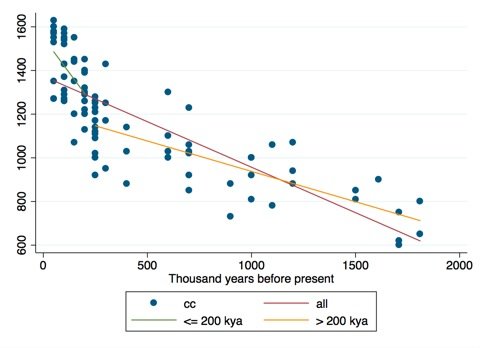
Another way to consider our data is to look at the residuals. The residuals are simply the difference between our true values and the best fit line of our model. A good way to think about residuals is to imagine rotating our data above anticlockwise until the best fit line is horizontal. Since a horizontal line has a slope of zero, it also has a zero correlation with the x-variable, in our example time. In so doing, we can consider the differences in the residuals, having controlled for time. When we compare the residuals using the best fit line the means for the two time periods (separated by a grey dashed line) are significantly different. The model is also statistically significant: t(84) = -3.9994, p < 0.001. The mean difference in cranial capacity between the two periods is 122 cc; a difference of 31%. This corresponds well with Blakemore's figure. However, it is important to note that this is the mean difference between the two periods and does not necessarily indicate an abrupt change at 200,000 years ago.
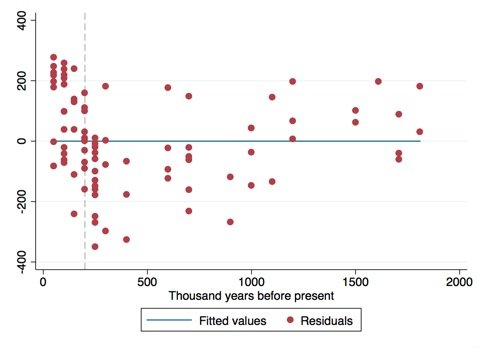
While the numbers seem to agree with the hypothesis of a marked increase in cranial size for the later period, I think the weight Blakemore gives it is rather foolish. The fossil record is patchy and likely unrepresentative of the true cranial variation of past hominins. As Jerry Coyne rightfully points out, a geologically sudden change in the fossil record may simply reflection how erratic it is. We already saw how cranial size can change markedly in 30,000 years – little more than a blip on the time scale that we are considering here. The gradual decrease in cranial capacity since the early Upper Palaeolithic would seem geologically sudden when considered on the above timescale. The size of the fossil record is small enough that the discovery of five or six new specimens could mean having to revise our figures once again.
Another problem is that calculating cranial capacity is not an exact science. While advances have been made in calculating cranial capacity, in many cases it should still be considered a best guess (de Miguel and Henneberg, 2001). This is particularly the case for palaeoanthropological material which tends to come out of the ground fragmented and deformed. With all its drawbacks, the fossil record is often all we have to answer some of our most pressing questions. At the same time, we need to always be conscious of what the record can and cannot tell us, and avoid the temptation to tell "fanciful tales".
References
De Miguel C and Henneberg M (2001) Variation in hominid brain size: how much is due to method? Homo 52: 3–58.
Lee S-H and Wolpoff MH (2003) The pattern of evolution in Pleistocene human brain size. Paleobiology 29:186-196.

The question is: why is [our brain] so big compared to the brains of our predecessors, such as Homo erectus? Until 200,000 years ago, there had been a gradual increase in brain size among hominins, starting three million years ago. Then, abruptly, there was a remarkable increase of about 30% or so.

John Hawks is not convinced that there is any abrupt change in cranial capacity. Referring to the above graph showing endocranial volume against time he writes:
As you can see, there's no sudden jump 200,000 years ago, or at any other time. The data, such as they are, are consistent with a single pattern of increase over time, as pointed out by Sang-Hee Lee and Milford Wolpoff (2003).
Heck, it's the lack of a sudden jump that has gotten all the attention. Because if "modern" humans suddenly showed up in Africa 200,000 years ago, and all of a sudden had vastly larger brains than any other hominins, wouldn't that be a simple and tidy story? Don't you think we'd all be talking about the sudden origin of modern humans as reflected by their larger brains?
It just didn't happen.
Heck, it's the lack of a sudden jump that has gotten all the attention. Because if "modern" humans suddenly showed up in Africa 200,000 years ago, and all of a sudden had vastly larger brains than any other hominins, wouldn't that be a simple and tidy story? Don't you think we'd all be talking about the sudden origin of modern humans as reflected by their larger brains?
It just didn't happen.
I decided to take the data from the Lee and Wolpoff paper and compare the periods prior and subsequent to 200,000 years ago. As Hawks eluded to, the data can be explained by a linear model. However, this is not very helpful since we can easily fit a line or curve to just about any data. More to the point, a single fitted line doesn't tell us much about any changes in the data. The red line in the graph below corresponds to the best fit line for the entire dataset (r = 0.81). The green and orange lines are the best fit lines for the two time periods we are considering. We can see that slopes of all three lines differ appreciably from one another. An analysis of covariance test confirms that there is a significant difference in cranial capacity between the two time periods, after we control for time. The model is statistically significant: F(1, 84) = 107, p < 0.001.

Another way to consider our data is to look at the residuals. The residuals are simply the difference between our true values and the best fit line of our model. A good way to think about residuals is to imagine rotating our data above anticlockwise until the best fit line is horizontal. Since a horizontal line has a slope of zero, it also has a zero correlation with the x-variable, in our example time. In so doing, we can consider the differences in the residuals, having controlled for time. When we compare the residuals using the best fit line the means for the two time periods (separated by a grey dashed line) are significantly different. The model is also statistically significant: t(84) = -3.9994, p < 0.001. The mean difference in cranial capacity between the two periods is 122 cc; a difference of 31%. This corresponds well with Blakemore's figure. However, it is important to note that this is the mean difference between the two periods and does not necessarily indicate an abrupt change at 200,000 years ago.

While the numbers seem to agree with the hypothesis of a marked increase in cranial size for the later period, I think the weight Blakemore gives it is rather foolish. The fossil record is patchy and likely unrepresentative of the true cranial variation of past hominins. As Jerry Coyne rightfully points out, a geologically sudden change in the fossil record may simply reflection how erratic it is. We already saw how cranial size can change markedly in 30,000 years – little more than a blip on the time scale that we are considering here. The gradual decrease in cranial capacity since the early Upper Palaeolithic would seem geologically sudden when considered on the above timescale. The size of the fossil record is small enough that the discovery of five or six new specimens could mean having to revise our figures once again.
Another problem is that calculating cranial capacity is not an exact science. While advances have been made in calculating cranial capacity, in many cases it should still be considered a best guess (de Miguel and Henneberg, 2001). This is particularly the case for palaeoanthropological material which tends to come out of the ground fragmented and deformed. With all its drawbacks, the fossil record is often all we have to answer some of our most pressing questions. At the same time, we need to always be conscious of what the record can and cannot tell us, and avoid the temptation to tell "fanciful tales".
References
De Miguel C and Henneberg M (2001) Variation in hominid brain size: how much is due to method? Homo 52: 3–58.
Lee S-H and Wolpoff MH (2003) The pattern of evolution in Pleistocene human brain size. Paleobiology 29:186-196.
Finger points to new human
Thu, Mar 25 2010 08:07 | Palaeoanthropology | Permalink

A team of archaeologists have found the bone of a little finger while digging at Denisova Cave in the Altai Mountain, located in southern Siberia. The size of the bone suggests that it came from a child between five and seven years of age. It is difficult to distinguish between different species of humans based solely on the morphology of a single finger bone. However, the tentative dates put the age of the bone at between 30,000 and 50,000 years ago; a window of time when both Neandertals and modern humans coexisted in Eurasia.
In order to determine which species the little finger came from, Johannes Krause of the Max Planck Institute for Evolutionary Anthropology in Leipzig, Germany sequenced the complete mitochondrial DNA (mtDNA) from the finger bone and compared its DNA with that of modern humans and Neandertals. What they found surprised everyone involved in the project. The mtDNA of Denisova did not match that of modern humans or Neandertals. In fact it last shared a common ancestor with us and Neandertals in Africa around a million years ago.
We know of three major hominin migrations out of Africa. The first occurred with Homo erectus around 1.9 million years ago, followed by the ancestors of Neandertals sometime between 500,000 and 300,000 years ago, and finally modern humans around 50,000 years ago. This makes the Denisova specimen too late to be part of the Homo erectus exodus and too early to part of the other two.
However, it may be a little premature to declare this a new species of human. Svante Pääbo's team is already busy sequencing the nuclear DNA of the Denisova specimen. One, albeit unlikely, possibility is that this will turn out to be a representative of an outlier Neandertal population. Previous studies have found a wide diversity in both the morphology and mitochondria of geographically separated Neandertal populations. Until the nuclear DNA has been mapped it is not possible to definitively say if we are really dealing with a new species of human. However, if this does turn out to be the case, it would mean that we shared the globe with at least three other species of humans as late as 40,000 years ago.
The incredible shrinking human brain
Sun, Mar 14 2010 05:12 | Palaeoanthropology | Permalink
The Times Online recently ran a story about a French team that have made an endocast by digitally scanning inside the skull of Cro-Magnon 1, perhaps the most famous of all Upper Palaeolithic skulls. The mould of Cro-Magnon highlights what has been long known about these European early modern humans since their first discovery in 1868 - they had bigger brains than us. In fact, average human brain size has been decreasing during the last 30,000 or so years. This revelation was rather troubling for nineteenth-century anthropologists who sought to link brain size with intelligence. Not only did these early modern humans have a larger brain volume than us, but so too did the Neanderthals, who were regarded by many at the time as “a barbarous and savage race” (Schaaffhausen 1858). To add injury to insult, the decrease in head size coincides with some of the greatest cultural innovations in human history.
The Times article forwards a number of the various hypotheses about why brain size has decreased. Antoine Balzeau reasons that “the cerebellum — a brain structure linked to language and concentration — appears to take up a larger proportion of the head now than in the time of Cro Magnon 1.” While it is true that the cerebellum is proportionally larger in modern humans, it is proportionally smaller than in apes, by around 20%. We still don’t know enough about brain function to be able to say what advantage, if any, a larger cerebellum would give us.
Second up, is the suggestion that big heads are somehow an adaptation to cold climate. There are a number of problems with this idea. If having a large skull is an adaptation to cold environments we would expect to see such traits peaking in the aftermath of the Last Glacial Maximum around 20,000 years ago, many millennia after Cro-Magnon walked the earth. As a general rule people living in Arctic regions tend to have more rounded heads, unlike the long headed Cro-Magnon. What’s more, the limbs of Cro-Magnon and their kin are quite long, contradicting Allen’s Rule which predicts that species will evolve smaller appendages as an adaptation to colder climes. Their body type also differs markedly from that of Neandertals, for whom there is a better case to made of being cold-adapted.
The article goes on to suggest diet as a driving force behind the decrease in head size. Cranial robusticity has indeed been shown to correlate with diet. It is important, however, to make the distinction between cranial size and robusticity. While the two are related they do not necessarily go hand in hand. While the Gravettian populations were undoubtedly more robust than most modern-day populations, they are not especially robust when compared to the Mesolithic populations of Téviec and Hoëdic or modern Aboriginal Australian or Fuegian populations. It is also unclear what dietary innovation could account for the decrease in head size. We have unambiguous evidence for the control of fire at around 250,000 years ago, while agriculture did not appear until around 10,000 years ago. The dates just don’t add up.
The article suggest one more hypothesis for the downsizing of the brain: “… with high infant mortality, only the toughest survived — and the toughest tended to have big heads.” Infant mortality is an ever-present problem for humans because bipedalism has constrained the size of the birth canal. If anything, giving birth to a larger headed children is going to lead to increased mortality for both the mother and child. Indeed, natural selection has restricted in utero brain growth in humans, with a large proportion of brain development occurring outside of the womb. In most non-human primates, the brain is close to adult size by the first year of life. In humans, on the other hand, near-adult brain size is not reached until about ten years of age.
Perhaps, the best explanation for the larger head size of our ancestors is one that the authors failed to mention – allometry. Bigger animals have bigger brains. While the cranial capacity for modern humans is large for a primate of our size, it is still only about a quarter of the size of that of an elephant. The decrease in brain size during the late Pleistocene was also accompanied by a decrease in body size. In other primates that show a decrease in brain size, there is an accompanying decrease in body size. Having a larger brain comes at a cost. The brain is a greedy glucose-guzzling tissue. The is possible that our smaller brain has allowed us to reallocate energy for other bodily functions.
References and further reading
Henneberg M. Evolution of the human brain: is bigger better?. Clin Exp Pharmacol Physiol 1998, 25:745-749.
Schaaffhausen H. On the crania of the most Ancient Races of Man. Müllers Archiv 1858:453.
Ruff C, Trinkaus E, Holliday T. Body mass and encephalisation in Pleistocene Homo. Nature 1997: 387: 173–6.

The Times article forwards a number of the various hypotheses about why brain size has decreased. Antoine Balzeau reasons that “the cerebellum — a brain structure linked to language and concentration — appears to take up a larger proportion of the head now than in the time of Cro Magnon 1.” While it is true that the cerebellum is proportionally larger in modern humans, it is proportionally smaller than in apes, by around 20%. We still don’t know enough about brain function to be able to say what advantage, if any, a larger cerebellum would give us.
Second up, is the suggestion that big heads are somehow an adaptation to cold climate. There are a number of problems with this idea. If having a large skull is an adaptation to cold environments we would expect to see such traits peaking in the aftermath of the Last Glacial Maximum around 20,000 years ago, many millennia after Cro-Magnon walked the earth. As a general rule people living in Arctic regions tend to have more rounded heads, unlike the long headed Cro-Magnon. What’s more, the limbs of Cro-Magnon and their kin are quite long, contradicting Allen’s Rule which predicts that species will evolve smaller appendages as an adaptation to colder climes. Their body type also differs markedly from that of Neandertals, for whom there is a better case to made of being cold-adapted.
The article goes on to suggest diet as a driving force behind the decrease in head size. Cranial robusticity has indeed been shown to correlate with diet. It is important, however, to make the distinction between cranial size and robusticity. While the two are related they do not necessarily go hand in hand. While the Gravettian populations were undoubtedly more robust than most modern-day populations, they are not especially robust when compared to the Mesolithic populations of Téviec and Hoëdic or modern Aboriginal Australian or Fuegian populations. It is also unclear what dietary innovation could account for the decrease in head size. We have unambiguous evidence for the control of fire at around 250,000 years ago, while agriculture did not appear until around 10,000 years ago. The dates just don’t add up.
The article suggest one more hypothesis for the downsizing of the brain: “… with high infant mortality, only the toughest survived — and the toughest tended to have big heads.” Infant mortality is an ever-present problem for humans because bipedalism has constrained the size of the birth canal. If anything, giving birth to a larger headed children is going to lead to increased mortality for both the mother and child. Indeed, natural selection has restricted in utero brain growth in humans, with a large proportion of brain development occurring outside of the womb. In most non-human primates, the brain is close to adult size by the first year of life. In humans, on the other hand, near-adult brain size is not reached until about ten years of age.
Perhaps, the best explanation for the larger head size of our ancestors is one that the authors failed to mention – allometry. Bigger animals have bigger brains. While the cranial capacity for modern humans is large for a primate of our size, it is still only about a quarter of the size of that of an elephant. The decrease in brain size during the late Pleistocene was also accompanied by a decrease in body size. In other primates that show a decrease in brain size, there is an accompanying decrease in body size. Having a larger brain comes at a cost. The brain is a greedy glucose-guzzling tissue. The is possible that our smaller brain has allowed us to reallocate energy for other bodily functions.
References and further reading
Henneberg M. Evolution of the human brain: is bigger better?. Clin Exp Pharmacol Physiol 1998, 25:745-749.
Schaaffhausen H. On the crania of the most Ancient Races of Man. Müllers Archiv 1858:453.
Ruff C, Trinkaus E, Holliday T. Body mass and encephalisation in Pleistocene Homo. Nature 1997: 387: 173–6.
Irish Neandertals!
Thu, Dec 3 2009 01:23 | Palaeoanthropology | Permalink
This abstract from a 1961 paper made me smile:
Casey AE, Franklin RB. 1961. Cork-kerry Irish compared anthropometrically with 139 modern and ancient peoples. Irish Journal of Medical Science. 36 (9).
Living Cork-Kerry Irish were compared with 139 modern and ancient peoples using 36 factors, 14 blood groups, 3 skin, hair and eye pigmentations and 22 physical measurements. The method was a form of multiple correlation in which the class interval for each factor was one-half the standard deviation, and numerical values allocated to each half-standard deviation. The Irish, Northern Scots, Icelanders, S.W. Norse, N. Dutch and Frisians form a racial entity with 97 per cent. inter-correlation and very little change during the past 1,000–4,000 years. There is a high correlation with the ancient Scythians substantiating the Irish legends of descent from the kings of Scythia. There is a substantial mixture of upper palaeolithic and Neanderthal man in the north-western perimeter of Europe, exemplified by the people of Cork and Kerry, a mixture not shared by the American Indians, the Australian Aborigines, and by the Bushmen and Pygmies of Africa. There is a good possibility that the large frame, red hair, blue eyes and white skin of West Europe was contributed by upper palaeolithic and Neanderthal men.
Casey AE, Franklin RB. 1961. Cork-kerry Irish compared anthropometrically with 139 modern and ancient peoples. Irish Journal of Medical Science. 36 (9).
One chin does not a modern human make
Sat, Nov 21 2009 01:04 | Palaeoanthropology | Permalink
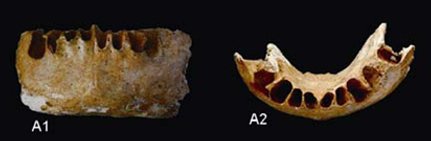
Chinese scientists say that a recently discovered partial jaw from Guangxi challenges the ‘out of Africa’ model of modern human origins, while lending support to the multiregional hypothesis. The 110,000 year-old mandible is described as having a chin that juts “ever so slightly outward.” These scientists assert that the presence of chin shows that there was significant gene flow between populations of modern Homo sapiens and archaic Homo.
Wu Xinzhi of the Chinese Academy of Sciences had the following to say about the find:
”The finding was strong evidence to prove the multiregional model, and from this evidence, it was significant to solve the academic dispute between 'the multiregional mode' and 'out of Africa theory’”.
It is interesting to note Xinzhi’s use of the past simple tense to suggest that this is a closed case. Far from it! Palaeoanthropological theory has moved on from the multiregional sensu stricto versus ‘out of Africa’ sensu stricto dichotomy that predominated the discussion during the latter half of the last century. Nevertheless, the question of how much gene flow, if any, took place between modern and archaic Homo is still very much a debated issue.At this stage you may be wondering why there has been such furore over a chinned jaw. As long ago as 1775, Johann Friedrich Blumenbach commented on the uniqueness of the modern human chin:
In the animals there is scarcely a particular chin which can be considered as comparable to that of man: and in those men who, as is often said, seem to have something apish in their countenance, this generally resides in a deeply-retreated chin.
The distinctive modern human chin develops through the combination of bone deposition on the inferior part of the jaw and resorption around the alveolar region. In other primates the entire jaw undergoes deposition. The modern human chin is characterised as having a central keel, with hollowed out depressions (known as mental fossae) to either side, together with a protruding inferior portion. This distended mental protuberance and lateral extremities make up the mental trigone, giving the chin the appearance of an inverted T. It is the combination of all these anatomical features that make up the prototypal modern chin. However, chins show great variability, with some modern humans not having any.This variability is also extends to earlier hominins. The Middle Pleistocene fossils from the Sima de los Huesos have been described as having chins, and even well-developed mental trigones. Among Pleistocene hominins, Neandertals appear to have the most divergent pattern from the modern configuration, universally lacking the inverted T and mental fossae. While it has been argued that the Neandertal mandibles from the Croatian site of Vindija show the development of incipient chins, this has not been borne out by later analyses.
Some of the ‘modern’ Klasies River Mouth mandibles do not have developed mental trigones, midline keel or a thickening of the inferior margin. However, the modern designation of this material is controversial with these fossils showing a mosaic of both archaic and modern features. Similarly, the modern humans from Qafzeh show variable expression of the inverted T and mental fossae, with no indication of these features in the Skhūl specimens. The 700-800,000 year-old Tighenif mandibles show a surprisingly modern configuration complete with central keel, a thickened inferior portion, and the development of a triangular protuberance. The presence of a chin in these specimens could represent a synapomorphy with modern humans.
Based on the archaeological record, it appears that modern humans left Africa some time around 100,000 years ago. Among the oldest undisputed modern human remains in China come from Zhoukoudian Cave at around 35,000 years BP. The possibly earlier fossil from Liujiang is marred with dating problems. In order for the Chinese scientists’ assertion to hold, it would require an even earlier exit from Africa or expansive gene flow between modern humans living in Africa and archaic humans in Asia; claims for which the evidence is currently lacking. Future analyses of the specimens will determine whether these chins have a truly modern form or whether the pattern is more like the non-homologous protruding inferior jaws seen in other archaic specimens. Alternatively, if these specimens end up being the result of convergent evolution it would raise questions about the functional significance of a chin. Finally, if these fossils show a pattern similar to the one seen in the Tighenif fossils it may suggest that they belong to the same clade.
References and further reading
Ahern JC (1993). The Transitional Nature of the Late Neandertal Mandibles from Vindija Cave, Croatia. M.A. thesis. Department of Anthropology, Northern Illinois University.
Blumenbach, JF (1978). The anthropological treatises of Johann Friedrich Blumenbach / translated and edited from the Latin, German, and French originals by Thomas Bendyshe. Boston : Longwood Press.
Hawks, J (2009). It came from Guangxi.
McKenna, P (2009). Chinese challenge to 'out of Africa' theory. New Scientist.
Rosas, A. (1995). Seventeen new mandibular specimens from the Atapuerca/Ibeas Middle Pleistocene hominids sample. J. hum. Evol. 28, 533–559.
Schwartz JH, Tattersall I (2000). The human chin revisited: what is it and who has it? J Hum Evol 38:367-409.
Schwartz JH, Tattersall I (2002) The Human Fossil Record, Vol. 1: Craniodental Morphology of Genus Homo (Europe) Wiley-Liss: New York.
Stone R (2009). Signs of Early Homo sapiens in China? Science 326 (5953) p 655.
Above image: Institute of Vertebrate Palaeontology and Palaeoanthropology, Chinese Academy of Sciences.
Full frontal hominins
Thu, Nov 5 2009 01:43 | Palaeoanthropology | Permalink
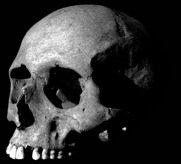
One of the most visually striking differences between modern humans and other hominins is the shape of the forehead. The frontal bone of the forehead serves two primary functions: it houses the frontal lobes of the brain in the anterior cranial fossa and also forms the orbital roof. When the orbits are positioned anterior to the frontal lobes, a supraorbital torus or brow ridge, forms in order to bridge the gap. This is particularly the case in archaic members of the genus Homo, whose brain cases are positioned well behind their faces.
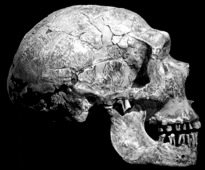
The incredible brow ridges of Homo erectus is perhaps this species most salient physical feature. They possess a flattened forehead with a bar-like brow ridge over the eye sockets. The supraorbital torus is continuous and thickened laterally, which in turn is associated with a pinching of the orbital breadth behind the eye sockets, known as postorbital constriction. In H. erectus, the supraorbital torus is separated from the frontal squama by a depression called the posttoral sulcus. While most Erectines conform to this general bauplan, there is a lot of regional variation in the exact form of the torus.
Neandertals are characterised by their long, large, low and wide skull. They have a double-arched browridge above the orbits, which angles backward on the sides of the face. It is depressed along the middle by the presence of a supraglabellar fossa. Compared to H. erectus, Neandertals have a more vertical and rounded forehead, with a less pronounced supraorbital torus.
Modern humans have a vertical forehead, due to in no small part to the expansion of the front part of the brain. Unlike in other hominins, the frontal lobes sit directly above the orbits, negating the need for a supraorbital torus. Instead, we tend to have relatively lightly developed superciliary arches. In present day populations, large supraorbitals are generally seen in individuals that have both robust and narrow skulls. Supraorbital ridges can also occur in cases of neurodevelopmental disorders, such as microcephaly, in which case normal orbital size is combined with smaller cerebral size. The presence of a supraorbital torus in the hominin Homo floresiensis was one of the traits that some researchers used to suggest that these dwarf humans were in fact microcephalic Homo sapiens.
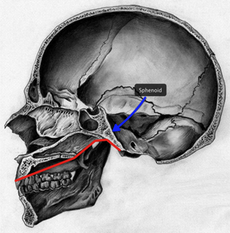
Modern adult humans have the most flexed basicranium of any mammal. This is due largely to us having a more vertically oriented sphenoid bone. A more flexed cranial base repositions the face directly below the anterior cranial fossa, while a more extended cranial base results in greater facial prognathism. In turn, the combination of an extended cranial base and facial forwardness influences the development of the supraorbital region. Early modern human skulls, such as Skhūl V and Dar es-Soltan, have prominent brow ridges. The development of large supraorbitals in these specimens results from greater cranial base angulation. In this regard, the development of the supraorbital region in some early modern humans does not result from neuro-orbital disjunction like in archaic humans, but primarily because of their more extended cranial base.
While much has been written about the non-metric variation of the frontal in hominins, there is little in the way of metric analyses, due to the bone's lack of cranial landmarks. Sheela Athreya recently carried out a quantitative study of the frontal bones of various Pleistocene hominins. She collected outlines along the sagittal and parasagittal planes of the bone. Based on her analyses, specimens were classified as either Early Pleistocene, Homo erectus, Middle Pleistocene, Neandertal or anatomically modern Homo sapiens.
The highest classification accuracy was along the midsagittal plane, with a success rate of a mere 68%. In other words, using this technique almost one-third of specimens were misclassified. A well-seasoned palaeoanthropologist would have a much higher success rate using only non-metric traits. The key to identifying which species a particular frontal bone comes from involves looking at the totality of features along the entire length of the torus and surrounding bone. It is likely that if each of the curves were combined in a multivariate analysis they would have yielded a much higher classificatory success rate. Linear measurements along a curve only capture two dimensions of the frontal form, thereby losing a lot of information contained in the third dimension. A better approach would be to digitise a three-dimensional dense point cloud along the entire bone and to analyse the region using geometric morphometrics. However, such equipment is expensive and not available in most anthropology departments.
Perhaps the most important outcome of this study was that it quantitatively confirmed some of the general characteristics of the frontal form of Homo, that have been previously described qualitatively. These include the fact that most of the variation in the frontal bone between Pleistocene groups is along the midsagittal plane. The study additionally found Homo erectus to differ from all other groups in the projection of the glabellar region. Finally, it identified modern humans as differing from all other groups in the curvature of the forehead, as well as the prominence of the lateral supraorbital torus. This confirms what many palaeoanthropologists have been saying for a long time – the lack of a supraorbital torus in modern humans is a uniquely derived feature.
References
Athreya, S. A comparative study of frontal bone morphology among Pleistocene hominin fossil groups, J Hum Evol (2009), doi:10.1016/j.jhevol.2009.09.003.
Lahr, MM. The Evolution of Modern Human Diversity : A Study on Cranial Variation . Cambridge; New York : Cambridge University Press, 1996.
Lieberman, Daniel E, Osbjorn M Pearson, and Kenneth M Mowbray. "Basicranial Influence on Overall Cranial Shape." Journal of Human Evolution 38 (2000): doi:10.1006/jhev.1999.0335.
Martin RD, MacLarnon AM, Phillips JL, Dussebieux L, Williams PR, Dobyns WV. 2006a. Comment on ‘‘The brain of LB1, Homo floresiensis.’’ Science 312:999b.
Trinkaus. Modern Human versus Neandertal Evolutionary Distinctiveness. Current Anthropology (2006) vol. 47 (4) pp. 597-620.
Trinkaus. European early modern humans and the fate of the Neandertals. Proceedings of the National Academy of Sciences (2007) 104 (18) pp. 7367-7372.
Above photos modified from originals by missmareck and arnybo under creative commons license.
Image of lateral dissected skull by dollinjune14, via deviantART (modified from original).

The incredible brow ridges of Homo erectus is perhaps this species most salient physical feature. They possess a flattened forehead with a bar-like brow ridge over the eye sockets. The supraorbital torus is continuous and thickened laterally, which in turn is associated with a pinching of the orbital breadth behind the eye sockets, known as postorbital constriction. In H. erectus, the supraorbital torus is separated from the frontal squama by a depression called the posttoral sulcus. While most Erectines conform to this general bauplan, there is a lot of regional variation in the exact form of the torus.
Neandertals are characterised by their long, large, low and wide skull. They have a double-arched browridge above the orbits, which angles backward on the sides of the face. It is depressed along the middle by the presence of a supraglabellar fossa. Compared to H. erectus, Neandertals have a more vertical and rounded forehead, with a less pronounced supraorbital torus.
Modern humans have a vertical forehead, due to in no small part to the expansion of the front part of the brain. Unlike in other hominins, the frontal lobes sit directly above the orbits, negating the need for a supraorbital torus. Instead, we tend to have relatively lightly developed superciliary arches. In present day populations, large supraorbitals are generally seen in individuals that have both robust and narrow skulls. Supraorbital ridges can also occur in cases of neurodevelopmental disorders, such as microcephaly, in which case normal orbital size is combined with smaller cerebral size. The presence of a supraorbital torus in the hominin Homo floresiensis was one of the traits that some researchers used to suggest that these dwarf humans were in fact microcephalic Homo sapiens.
Modern adult humans have the most flexed basicranium of any mammal. This is due largely to us having a more vertically oriented sphenoid bone. A more flexed cranial base repositions the face directly below the anterior cranial fossa, while a more extended cranial base results in greater facial prognathism. In turn, the combination of an extended cranial base and facial forwardness influences the development of the supraorbital region. Early modern human skulls, such as Skhūl V and Dar es-Soltan, have prominent brow ridges. The development of large supraorbitals in these specimens results from greater cranial base angulation. In this regard, the development of the supraorbital region in some early modern humans does not result from neuro-orbital disjunction like in archaic humans, but primarily because of their more extended cranial base.
While much has been written about the non-metric variation of the frontal in hominins, there is little in the way of metric analyses, due to the bone's lack of cranial landmarks. Sheela Athreya recently carried out a quantitative study of the frontal bones of various Pleistocene hominins. She collected outlines along the sagittal and parasagittal planes of the bone. Based on her analyses, specimens were classified as either Early Pleistocene, Homo erectus, Middle Pleistocene, Neandertal or anatomically modern Homo sapiens.
The highest classification accuracy was along the midsagittal plane, with a success rate of a mere 68%. In other words, using this technique almost one-third of specimens were misclassified. A well-seasoned palaeoanthropologist would have a much higher success rate using only non-metric traits. The key to identifying which species a particular frontal bone comes from involves looking at the totality of features along the entire length of the torus and surrounding bone. It is likely that if each of the curves were combined in a multivariate analysis they would have yielded a much higher classificatory success rate. Linear measurements along a curve only capture two dimensions of the frontal form, thereby losing a lot of information contained in the third dimension. A better approach would be to digitise a three-dimensional dense point cloud along the entire bone and to analyse the region using geometric morphometrics. However, such equipment is expensive and not available in most anthropology departments.
Perhaps the most important outcome of this study was that it quantitatively confirmed some of the general characteristics of the frontal form of Homo, that have been previously described qualitatively. These include the fact that most of the variation in the frontal bone between Pleistocene groups is along the midsagittal plane. The study additionally found Homo erectus to differ from all other groups in the projection of the glabellar region. Finally, it identified modern humans as differing from all other groups in the curvature of the forehead, as well as the prominence of the lateral supraorbital torus. This confirms what many palaeoanthropologists have been saying for a long time – the lack of a supraorbital torus in modern humans is a uniquely derived feature.
References
Athreya, S. A comparative study of frontal bone morphology among Pleistocene hominin fossil groups, J Hum Evol (2009), doi:10.1016/j.jhevol.2009.09.003.
Lahr, MM. The Evolution of Modern Human Diversity : A Study on Cranial Variation . Cambridge; New York : Cambridge University Press, 1996.
Lieberman, Daniel E, Osbjorn M Pearson, and Kenneth M Mowbray. "Basicranial Influence on Overall Cranial Shape." Journal of Human Evolution 38 (2000): doi:10.1006/jhev.1999.0335.
Martin RD, MacLarnon AM, Phillips JL, Dussebieux L, Williams PR, Dobyns WV. 2006a. Comment on ‘‘The brain of LB1, Homo floresiensis.’’ Science 312:999b.
Trinkaus. Modern Human versus Neandertal Evolutionary Distinctiveness. Current Anthropology (2006) vol. 47 (4) pp. 597-620.
Trinkaus. European early modern humans and the fate of the Neandertals. Proceedings of the National Academy of Sciences (2007) 104 (18) pp. 7367-7372.
Above photos modified from originals by missmareck and arnybo under creative commons license.
Image of lateral dissected skull by dollinjune14, via deviantART (modified from original).
Did Neandertals and modern humans interbreed?
Tue, Oct 27 2009 11:36 | Palaeoanthropology | Permalink
Ever since William King proposed the taxonomic designation Homo neanderthalensis in 1864, there has been intense debate as to whether Neandertals represent a distinct species from us. Species, as defined by the biological species concept, are populations of organisms that can potentially interbreed and have fertile offspring. It is believed that the lineage leading to Neandertals and modern humans split sometime around 500,000 years ago. For most of their existence Neandertals and early modern humans were geographically isolated (and by extension reproductively isolated) from one another. The big question is whether they could have produced viable offspring when they met.
Today, most researchers acknowledge that some sexual encounters could have occurred between Neandertals and modern humans. The more interesting question is how common were these encounters and did they leave their mark on the modern gene pool. Undoubtedly, modern humans and Neandertals would have recognised each other as fellow humans but this does not mean that they would have acted humanely to each another. Countless social and psychological studies have shown humans to have a very strong "us versus them" mentality, that no doubt also existed in our ancestors. It is unlikely that modern humans and Neandertals had an easy relationship. Most sexual encounters that took place between the two were likely opportunistic and probably involved enslavement and rape.
The morphological evidence
Palaeoanthropologists generally have little problem seperating Neandertals and modern humans based on their gross morphologies. However, some of the earliest modern humans from central Europe have traits that have been seen as evidence for continuity between them and Neandertals. These fossils, particularly those from Peştera cu Oase in Romania and Mladeč in the Czech Republic, have been touted as exemplars for modern-Neandertal admixture. These specimens show traits that are seen in high frequencies in Neandertals, such as bunning of the occipital and the presence of a suprainiac fossa.
However, many researchers have questioned whether these traits are in fact distinctly Neandertal. For instance, the form of the occipital seems to be different in early Upper Palaeolithic populations, leading many to favour the term hemibun to describe the shape of the occipital in early Europeans. Lieberman and colleagues has gone as far as to suggest that the buns seen in these two groups are not homologous. Similarly, it has been argued that the shape of the suprainiac fossa is distinct in early modern Europeans compared to Neandertals.
A palpable difficulty in assessing proposed Neandertal traits in early modern humans is that both groups shared similar niches and some traits may be the result of lifetime behavioural adaptations or convergent evolution. Indeed, the shared robustness of these early humans is likely due to the higher physical activities of these Late Pleistocene groups than during later period.
The genetic evidence
Mitochondrial DNA (mtDNA) has some characteristics that make it ideal for analyses of ancient specimens. MtDNA is found in abundance – cells can have thousands of copies of mtDNA, while only containing two copies of nuclear DNA. Moreover, its structure and location within the cell make it more resistant to decay. All the studies of Neandertal mtDNA to date cluster outside the range for modern human mtDNA variation. However, the mitochondria contain only a small part of the total DNA that make up a genome. The possibility that Neandertal genes could show up somewhere else in the genome cannot be ruled out.
The recent announcement by Svante Pääbo that he is sure that Neandertals and modern humans had sex is quite a bold pronouncement coming from a scientist. It raises the question of whether this ascertain is based on some hard evidence they found while sequencing the Neandertal genome. It is possible that if there was some Neandertal genes passed on to the first moderns in Europe, they could have got eliminated from the subsequent gene pool as population sizes fluctuated during the more severe climatic episodes. A more likely scenario is that Pääbo's team found evidence of modern introgression in the Neandertal genome. In all likelihood the incoming modern humans were more numerous than the Neandertals, thereby absorbing the endemic populations through genetic swamping.
References
Caspari RE. 1991. The evolution of the posterior cranial vault in the central European Upper Pleistocene. PhD dissertation. Ann Arbor, MI: University of Michigan.
King, W., 1864. The reputed fossil man of Neanderthal. Quarterly Journal of Science 1, 88–97.
Krings et al. 1997. Neandertal DNA sequences and the origin of modern humans. Cell vol. 90 (1) pp. 19-30.
Krings M, Capelli C, Tschentscher F, et al. 2000. A view of Neandertal genetic diversity. Nat Genet 26, 144–146.
Lieberman et al. 2000. Basicranial influence on overall cranial shape. J. Hum. Evol. vol. 38 (2) pp. 291-315.
Nara MT. 1994. Etude de la variabilité de certainscaractères métriques et morphologiques des Néandertaliens. Bordeaux: Thèse de Docteur.
Pääbo S, Poinar H, Serre D, et al. 2004. Genetic analyses from ancient DNA. Ann Rev Genet 38, 645–679.

Above photos modified from originals by erix! and fangleman under creative commons license.
Today, most researchers acknowledge that some sexual encounters could have occurred between Neandertals and modern humans. The more interesting question is how common were these encounters and did they leave their mark on the modern gene pool. Undoubtedly, modern humans and Neandertals would have recognised each other as fellow humans but this does not mean that they would have acted humanely to each another. Countless social and psychological studies have shown humans to have a very strong "us versus them" mentality, that no doubt also existed in our ancestors. It is unlikely that modern humans and Neandertals had an easy relationship. Most sexual encounters that took place between the two were likely opportunistic and probably involved enslavement and rape.
The morphological evidence
Palaeoanthropologists generally have little problem seperating Neandertals and modern humans based on their gross morphologies. However, some of the earliest modern humans from central Europe have traits that have been seen as evidence for continuity between them and Neandertals. These fossils, particularly those from Peştera cu Oase in Romania and Mladeč in the Czech Republic, have been touted as exemplars for modern-Neandertal admixture. These specimens show traits that are seen in high frequencies in Neandertals, such as bunning of the occipital and the presence of a suprainiac fossa.
However, many researchers have questioned whether these traits are in fact distinctly Neandertal. For instance, the form of the occipital seems to be different in early Upper Palaeolithic populations, leading many to favour the term hemibun to describe the shape of the occipital in early Europeans. Lieberman and colleagues has gone as far as to suggest that the buns seen in these two groups are not homologous. Similarly, it has been argued that the shape of the suprainiac fossa is distinct in early modern Europeans compared to Neandertals.
A palpable difficulty in assessing proposed Neandertal traits in early modern humans is that both groups shared similar niches and some traits may be the result of lifetime behavioural adaptations or convergent evolution. Indeed, the shared robustness of these early humans is likely due to the higher physical activities of these Late Pleistocene groups than during later period.
The genetic evidence
Mitochondrial DNA (mtDNA) has some characteristics that make it ideal for analyses of ancient specimens. MtDNA is found in abundance – cells can have thousands of copies of mtDNA, while only containing two copies of nuclear DNA. Moreover, its structure and location within the cell make it more resistant to decay. All the studies of Neandertal mtDNA to date cluster outside the range for modern human mtDNA variation. However, the mitochondria contain only a small part of the total DNA that make up a genome. The possibility that Neandertal genes could show up somewhere else in the genome cannot be ruled out.
The recent announcement by Svante Pääbo that he is sure that Neandertals and modern humans had sex is quite a bold pronouncement coming from a scientist. It raises the question of whether this ascertain is based on some hard evidence they found while sequencing the Neandertal genome. It is possible that if there was some Neandertal genes passed on to the first moderns in Europe, they could have got eliminated from the subsequent gene pool as population sizes fluctuated during the more severe climatic episodes. A more likely scenario is that Pääbo's team found evidence of modern introgression in the Neandertal genome. In all likelihood the incoming modern humans were more numerous than the Neandertals, thereby absorbing the endemic populations through genetic swamping.
References
Caspari RE. 1991. The evolution of the posterior cranial vault in the central European Upper Pleistocene. PhD dissertation. Ann Arbor, MI: University of Michigan.
King, W., 1864. The reputed fossil man of Neanderthal. Quarterly Journal of Science 1, 88–97.
Krings et al. 1997. Neandertal DNA sequences and the origin of modern humans. Cell vol. 90 (1) pp. 19-30.
Krings M, Capelli C, Tschentscher F, et al. 2000. A view of Neandertal genetic diversity. Nat Genet 26, 144–146.
Lieberman et al. 2000. Basicranial influence on overall cranial shape. J. Hum. Evol. vol. 38 (2) pp. 291-315.
Nara MT. 1994. Etude de la variabilité de certainscaractères métriques et morphologiques des Néandertaliens. Bordeaux: Thèse de Docteur.
Pääbo S, Poinar H, Serre D, et al. 2004. Genetic analyses from ancient DNA. Ann Rev Genet 38, 645–679.
Above photos modified from originals by erix! and fangleman under creative commons license.
Homo heidelbergensis and the muddle in the middle
Sat, Oct 10 2009 11:45 | Palaeoanthropology | Permalink
At the conference, much attention was focused on the Middle Pleistocene "muddle in the middle" [3], particularly the role of Homo heidelbergensis in hominin evolution. While H. heidelbergensis possesses both archaic and derived traits intermediate between H. erectus and later members of the Homo genus, it lacks uniquely derived traits or autapomorphies, which are a prerequisite for defining a species.
H. heidelbergensis has traits that have been interpreted as nascent Neandertal autapomorphies, leading some researchers to propose that there was a continuous evolution of Neandertals [4-6]. This accretion model would make H. heidelbergensis a chronospecies on the continuum of the Neandertal lineage, a view championed by Jean-Jacques Hublin. The accretion model proposes that Neandertals evolved by anagenesis, i.e. non-branching evolutionary change.
Another scenario views both the European and African H. heidelbergensis as a single species, and the last common ancestor of both Neandertals and modern humans. Alternatively, H. heidelbergensis could have become isolated in Europe and evolved into Neandertals, while the African populations led to modern humans.
During the conference, Ian Tattersall noted that while the accretion model explains some of the variation in the Middle Pleistocene, it cannot account for some outliers, such as the 28 or so specimens that have been recovered from the Sima de los Huesos in Atapuerca, Spain. Tattersall is not the first author to call the accretion model into question [7]. Recent dates have placed the Sima fossils at just over half-a-million years old. Based on the dissimilarity between these fossils and the penicontemporaneous H. heidelbergensis from the rest of Europe, Tattersall proposes that two hominin lineages coexisted in Europe before the arrival of H. sapiens. He suggests that one line (which may include the Sima specimens) led to the Neandertals, while the branch which included H. heidelbergensis went extinct. If Tattersall is correct it would mean that the Sima fossils, which are currently classified as H. heidelbergensis, must be designated another name.
Hublin is to his guns and doesn't see any need to reclassify the Sima material. He goes as far as to suggest binning the species name H. heidelbergensis altogether and instead reassigning all these Middle Pleistocene fossils as H. neanderthalensis. Whatever the outcome is in this debate, it appears that hominin evolution in the Middle Pleistocene is more complex than we have previously suspected.
References
1. Balter M. New work may complicate history of Neandertals and H. sapiens. Science 2009; 326:224-5.
2. Darwin C. The descent of man, and selection in relation to sex. New York, A. L. Burt; 1874.
3. Butzer KW, Isaac GL, International Congress of Anthropological and Ethnological Sciences 9C1. After the Australopithecines : stratigraphy, ecology, and culture, change in the Middle Pleistocene . The Hague : Mouton ; Chicago : distributed in the USA and Canada by Aldine; 1976.
4. Hublin. Paleogeography, and the evolution of the Neandertals. In: Akazawa, Aoki, Bar-Yosef, Eds. Neandertals and Modern Humans in Western Asia. New York: Plenum Press; 1998:295-310.
5. Hublin. Climatic Changes, Paleogeography, and the Evolution of the Neandertals. In: Akazawa, Aoki, Bar-Yosef, Eds. Neandertals and Modern Humans in Western Asia. New York: Plenum Press; 1998:295-310.
6. Martinón-Torres M, Bastir M, Bermúdez de Castro JM, Gómez A, Sarmiento S, Muela A, Arsuaga JL. Hominin lower second premolar morphology: evolutionary inferences through geometric morphometric analysis. J Hum Evol 2006; 50:523-33.
7. Hawks JD, Wolpoff MH. The accretion model of Neandertal evolution. Evolution 2001; 55:1474-85.
The pelvis of Ardipithecus ramidus
Fri, Oct 2 2009 06:04 | Palaeoanthropology | Permalink
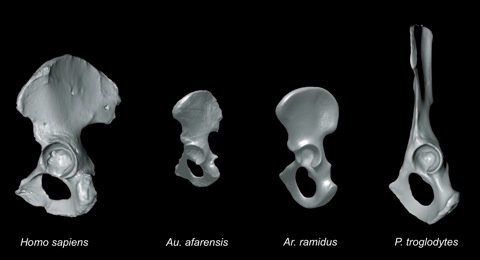
One of the anatomical features that sets humans apart from other living primates is the shape of our pelvis. The shift from a quadrupedal aboreal lifestyle to habitually walking on two legs requires a substantial reconfiguration of the hip region. The 4.4 million year old Ardipithecus ramidus fossil remains give us a glimpse of what the one of the earliest members of the hominin lineage looked like. While the feet of Ar. ramidus show that it was still adapted to life in the trees, the pelvis shows significant adaptations to walking upright on two legs.
The gluteus maximus, which is a relatively minor muscle in quadrupeds has been reconfigured into the largest muscle in humans, in order to stabilize the pelvis and trunk in an upright position. The derived nature of the ilium of Ar. ramidus suggests that the enlargement of the gluteal maximus had already begun. The craniocaudal height of the pelvis is also reduced, which would have lowered the relatively long trunk's centre of mass. This would have allowed for more stable bipedal locomotion.
However, the ischium is quite primitive compared to the ilia, likely to accommodate the large hindlimb musculature required for tree climbing. The two best preserved australopithicine pelves, AL 288-1 and Sts 14, both have short ischia, like those seen in modern humans. The preserved portion of the ischial ramus in Ar. ramidus is significantly larger than that found in any of the Australopithecines. A long ischium creates a greater moment arm suggesting that Ar. ramidus had relatively powerful hamstrings, a trait that is common in tree-dwelling primates.
The configuration of the ARA-VP-6/500 pelvis suggests that lower lumbars were probably posteriorly positioned, allowing for lordosis of the spine. A reduction in iliac height would have further facilitated lordosis. Lordosis positions the spine to a more forward position, so that it directly overlies the hips during erect posture. Lower spinal lordosis would have allowed the full extension of the hips and knee during extended bipedal locomotion.
Ar. ramidus was quite capable of bipedal locomotion, as attested to by the morphology of its pelvis and foot. However, its large thigh muscles and its prehensile big toe show that it was still very much adapted to arboreal life. Ar. ramidus shares arboreal adaptations that were probably present in the human-chimp last common ancestor, as well as bipedal adaptations that are so characteristic of hominins. Ar. ramidus appears to have been an arboreal ape with bipedal adaptations, rather than a biped with arboreal adaptations. It is not until almost half-a-million years later, with the arrival of Australopithecus afarensis, that we find a truly habitual bipedal hominin.
Fossil and data access in palaeoanthropology
Tue, Sep 8 2009 01:50 | Palaeoanthropology | Permalink
A recent article in Scientific American has generated a lot of buzz in the anthropology blogosphere. The piece discusses the problems of accessing human fossil remains, reopening the discussion on how open anthropology needs to be. The reason why data acquisition is such a problem in palaeoanthropology is captured in the opening sentence of an article Stephen Jay Gould and David Pilbeam wrote for Science:
Whenever supply cannot keep up with demand, you can be sure that problems will follow. (Many parents have learned this to their chagrin, when they find out that the Christmas toy du jour, their beloved child so wanted, is sold out.) Each newly unearthed fragment of human bone represents yet another valuable piece in the ever-growing jigsaw puzzle that is our evolutionary history. The study of primary data is of prime importance in paleoanthropology. As a result, a conflict arises, due to the need to study fossils and the limited access placed upon them. Restricted access occurs for a number of reasons, ranging from valid concerns over the fragility of a particular specimen, to scientists reaping the benefits of a research monopoly.
There is an unwritten rule in palaeoanthropology that the discoverers of a fossil have the exclusive rights to publish the initial monograph describing their specimen. Palaeoarchaeologists invest a lot of resources, time and effort in recovering fossils. They will often literally risk body and limb. Dehydration, food poisoning, snake bites, diseases and infections are but some of the hazards field archaeologists face. When they are not digging they are often engaged in the unenviable task of writing grants for their projects. It is understandable that they are wary of outsiders who expect free access to their hard-won prizes.
Ancient fossils usually come out of the ground highly fragmented and in a poor state of preservation. Much time is required to clean, preserve and reconstruct them before conducting a phylogenetic analysis. While many people have focused on the fact that certain specimens have taken an exhorbitant amount of time to describe, thus holding up the process of peer validation, it must also be kept in mind that these represent only a small fraction of the total human fossil record. While it of the utmost importance to make fossils available to outside investigators in a timely fashion, it is perhaps not the most fruitful or constructive area in which to be directing our attention.
Conflicts arise between researchers who want to access fossil material and curators who are genuinely concerned about the wear and tear that these fossils have endured through repeated handling. Curators will often direct researchers to others who have already measured the material in question, to avoid the redundant repetition of measurements. It is often at this point that researchers can come up against a brick wall, with peers who are unwilling to relinquish their valued data. Like the fossils themselves, unique data is a precious commodity and alas is necessary for publication. For good or for ill, peer-reviewed publications are placed in high regard in the anthropological world. Its role when it comes to job-seeking or tenure cannot be underestimated. An incredible amount of data has been collected through the years on ancient human remains but they are rarely put in the public domain. A noteworthy exception is the data on some 3,000 skulls from 17 worldwide populations, measured and made freely available by the eminent anthropologist William W. Howells (pdf file). The Howells' dataset is perhaps that man's most lasting legacy, at least in the sheer number of times his data have been used and referenced. Similarly, we need to place great value on other researchers who make their data available and this should be taken into consideration in matters of career advancement. At a minimum, the sharing of data should be deemed equivalent to research publication.
Positive steps have been taken in the ensure more data is made available. The US National Science Foundation encourage applicants to make provisions to make data available after the research has been completed. The NSF states that:
Anthropologists who fail to comply with these recommendations may have subsequent grant proposals turned down on these grounds. There is an ever-growing number of high quality casts and 3D images of fossils becoming available. Taphonomic processes may deform the fossilised bone and filling in gaps has often required a liberal amount of guesswork. 3D images often allow for better reconstructions of the original specimens, due to the ability to interpolate absent regions and more readily pinpoint and correct deformation. Research centres have woken up to the fact that collaborative projects tend to have a greater synergy due to their symbiotic nature. For palaeoanthropology to become a truly open discipline, it will not only need researchers to be more freehanded with their data, but will require funding agencies, universities and research centres to incentivise such actions.
Related reading
Fossil access editorial @ John Hawks weblog.
Science Suffers From The Idiots At Scientific American @ Anthropology.net.
Take your time @ A Primate of Modern Aspect.
Delson et al. Databases, data access, and data sharing in paleoanthropology: First steps. Evol. Anthropol. (2007) vol. 16 (5).
Gibbons. Glasnost for Hominids: Seeking Access to Fossils. Science (2002) vol. 297 pp. 1464-1468.
Mafart. Human fossils and paleoanthropologists: a complex relation. Journal of Anthropological Sciences (2008) vol. 86 pp. 201-204.
Pilbeam and Gould. Size and Scaling in Human Evolution. Science (1974) vol. 186 ( 4167), 892-901.
Tattersall and Schwartz. Is paleoanthropology science? Naming new fossils and control of access to them. Anat Rec (2002) vol. 269 (6) pp. 239-41.

"Human paleontology shares a peculiar trait with such disparate subjects as theology and extraterrestrial biology: it contains more practitioners than objects for study."
– Stephan J. Gould and David Pilbeam
Whenever supply cannot keep up with demand, you can be sure that problems will follow. (Many parents have learned this to their chagrin, when they find out that the Christmas toy du jour, their beloved child so wanted, is sold out.) Each newly unearthed fragment of human bone represents yet another valuable piece in the ever-growing jigsaw puzzle that is our evolutionary history. The study of primary data is of prime importance in paleoanthropology. As a result, a conflict arises, due to the need to study fossils and the limited access placed upon them. Restricted access occurs for a number of reasons, ranging from valid concerns over the fragility of a particular specimen, to scientists reaping the benefits of a research monopoly.
There is an unwritten rule in palaeoanthropology that the discoverers of a fossil have the exclusive rights to publish the initial monograph describing their specimen. Palaeoarchaeologists invest a lot of resources, time and effort in recovering fossils. They will often literally risk body and limb. Dehydration, food poisoning, snake bites, diseases and infections are but some of the hazards field archaeologists face. When they are not digging they are often engaged in the unenviable task of writing grants for their projects. It is understandable that they are wary of outsiders who expect free access to their hard-won prizes.
Ancient fossils usually come out of the ground highly fragmented and in a poor state of preservation. Much time is required to clean, preserve and reconstruct them before conducting a phylogenetic analysis. While many people have focused on the fact that certain specimens have taken an exhorbitant amount of time to describe, thus holding up the process of peer validation, it must also be kept in mind that these represent only a small fraction of the total human fossil record. While it of the utmost importance to make fossils available to outside investigators in a timely fashion, it is perhaps not the most fruitful or constructive area in which to be directing our attention.
Conflicts arise between researchers who want to access fossil material and curators who are genuinely concerned about the wear and tear that these fossils have endured through repeated handling. Curators will often direct researchers to others who have already measured the material in question, to avoid the redundant repetition of measurements. It is often at this point that researchers can come up against a brick wall, with peers who are unwilling to relinquish their valued data. Like the fossils themselves, unique data is a precious commodity and alas is necessary for publication. For good or for ill, peer-reviewed publications are placed in high regard in the anthropological world. Its role when it comes to job-seeking or tenure cannot be underestimated. An incredible amount of data has been collected through the years on ancient human remains but they are rarely put in the public domain. A noteworthy exception is the data on some 3,000 skulls from 17 worldwide populations, measured and made freely available by the eminent anthropologist William W. Howells (pdf file). The Howells' dataset is perhaps that man's most lasting legacy, at least in the sheer number of times his data have been used and referenced. Similarly, we need to place great value on other researchers who make their data available and this should be taken into consideration in matters of career advancement. At a minimum, the sharing of data should be deemed equivalent to research publication.
Positive steps have been taken in the ensure more data is made available. The US National Science Foundation encourage applicants to make provisions to make data available after the research has been completed. The NSF states that:
It expects investigators to share with other researchers, at no more than incremental cost and within a reasonable time, the data, samples, physical collections and other supporting materials created or gathered in the course of the work.
Anthropologists who fail to comply with these recommendations may have subsequent grant proposals turned down on these grounds. There is an ever-growing number of high quality casts and 3D images of fossils becoming available. Taphonomic processes may deform the fossilised bone and filling in gaps has often required a liberal amount of guesswork. 3D images often allow for better reconstructions of the original specimens, due to the ability to interpolate absent regions and more readily pinpoint and correct deformation. Research centres have woken up to the fact that collaborative projects tend to have a greater synergy due to their symbiotic nature. For palaeoanthropology to become a truly open discipline, it will not only need researchers to be more freehanded with their data, but will require funding agencies, universities and research centres to incentivise such actions.
Related reading
Fossil access editorial @ John Hawks weblog.
Science Suffers From The Idiots At Scientific American @ Anthropology.net.
Take your time @ A Primate of Modern Aspect.
Delson et al. Databases, data access, and data sharing in paleoanthropology: First steps. Evol. Anthropol. (2007) vol. 16 (5).
Gibbons. Glasnost for Hominids: Seeking Access to Fossils. Science (2002) vol. 297 pp. 1464-1468.
Mafart. Human fossils and paleoanthropologists: a complex relation. Journal of Anthropological Sciences (2008) vol. 86 pp. 201-204.
Pilbeam and Gould. Size and Scaling in Human Evolution. Science (1974) vol. 186 ( 4167), 892-901.
Tattersall and Schwartz. Is paleoanthropology science? Naming new fossils and control of access to them. Anat Rec (2002) vol. 269 (6) pp. 239-41.
Above photo by Simon Strandgaard under creative commons license.
Upper Palaeolithic Italy
Sun, Aug 23 2009 05:21 | Palaeoanthropology | Permalink
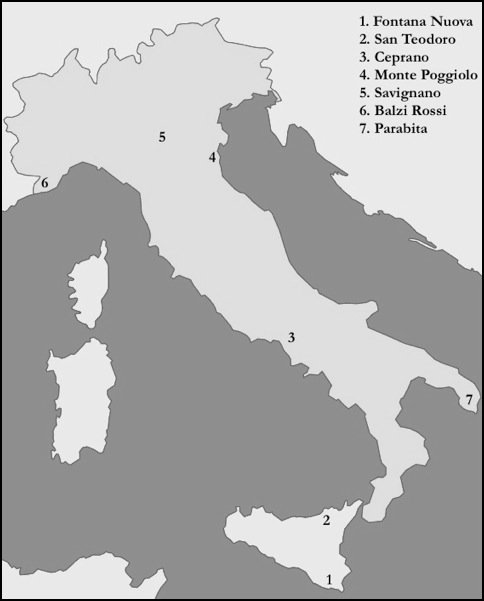
Extrapolating from data by Bocquet-Appel et al. (2005) it is possible that the population of Italy did not exceed 1,000 inhabitants. While this figure is little more than a best guess, it still gives us an idea of the fragile nature of these groups, who were living constantly on the edge of extinction. With such small numbers inbreeding can become a serious problem. However, studies of European Upper Palaeolithic people find them to be surprisingly homogenous as a whole, which would suggest a significant amount of gene flow between far-flung populations (Henke 1989, 1991). In order to understand how mating systems would have operated during the Early Upper Palaeolithic researchers have looked to modern hunter-gatherer groups. Such studies have found hunter-gatherers to have less group closure than agriculturalists. This would have been an important survival strategy for the hunter-gatherer groups of the Upper Palaeolithic, especially when faced with unpredictable environmental conditions and already constrained mating networks. This open network is also reflected in the material culture from this period. Venus figurines, dating chiefly from the Gravettian period, have been found over an area of approximately 2000 km, spanning from modern day Russia in the Northeast to Spain in the Southwest. The Italian Venuses from Savignano, Balzi Rossi and Parabita show the same female form, with an enlarged midsection, pronounced buttocks, large breasts and voluminous thighs, characteristic of the vast majority of these figurines. This suggests that there was an extensive flow of people and ideas during the Early Upper Palaeolithic. This is further supported by the transport of exotic materials, such as flint and shells over distances of several hundred kilometres.
At around 29,000 years ago, the Italian archaeological record falls silent. This hiatus seems to last for around two millennia. Mussi (2000) suggests that Italy may have become a "demographic trap", due to the combination of low population numbers and small group sizes, leading to the eventual extinction of the Aurignacian people.
Based on their assemblages, is believed that the Gravettian people were a new group, who likely entered the Italian peninsula from south-eastern France. As climatic conditions worsen, more exotic artefacts begin to appear in the archaeological record. A major concentration of sites date to around 25,000 BP, suggesting the arrival of immigrants from northern Europe coinciding with the onset of the Last Glacial Maximum. As new people entered Italy, they had to adapt to strange environments and a more limited variety of animals. We no longer find high altitude sites like in the Aurignacian. This shift to lower altitudes is undoubtedly due to the advancing ice. There is evidence of decreased mobility during the Last Glacial Maximum, which continues until the Mesolithic period (Holt 2003). This may reflect a move to more closed systems, as regionalisation grew due to the ever-increasing population density and greater competition over territories. The burial practices, art, and personal adornments of the Early Upper Palaeolithic, which paralleled those from the rest of the continent disappear at the height of the Last Glacial Maximum, further reflecting a shift to more closed social networks. As temperatures begin to improve towards the end of the Last Glacial Maximum, these cultural artefacts reappear in abundance, once again mirroring the styles and practices seen elsewhere in Europe.
Sicily comes in for special attention. The island has long been of interest to anthropologists, who saw the it as a stepping stone between North Africa and Europe. Ferembach (1986) postulated that Sicily may have served as an entry point for the North African Aterians (or their ancestors) around 50,000 years ago, who later became the "Cro-Magnon race". However, this idea finds little archaeological or skeletal support. Evidence of occupation prior to the Last Glacial Maximum in Sicily is patchy at best. At the site of Fontana Nuova, Aurignacian tools and isolated fragments of human bone have been found and estimated to date to around 30,000 years ago, making it the earliest record of occupation for the island (Chilardi et al 1996). However, it is the only evidence we have for the colonisation of the island during the Aurignacian and it is not until the Epigravettian (~20,000-10,000 BP) that we have further evidence of humans in Sicily. This suggests that the earliest settlers probably went extinct due their small numbers, limited resources and restricted gene flow with the mainland. It also reflects the pattern seen in the rest of Italy, since there are no Aurignacian sites known after 30,000 BP.
At the site of Grotta di San Teodoro the skeletal remains of seven individuals were found, making it the single largest Upper Palaeolithic sample in Sicily. An unpublished direct AMS 14C date situates the skeletal remains at around 14,800 BP (D’Amore et al. 2009). A recent craniometric study of the San Teodoro skeletons shows that they have higher affinities with the Late Upper Palaeolithic and Mesolithic populations, rather than Early Upper Palaeolithic ones (ibid.). These results suggest one of two possible scenarios (a) an early arrival with gene flow, thus explaining the homogeneity with the mainland Italian groups or (b) the late arrival of the direct descendants of the San Teodoro population on the island of Sicily. At the time of the Last Glacial Maximum there was probably a land bridge between Sicily and the Italian mainland, with sea levels being some 120 metres lower. The appearance of exotic faunal evidence further suggest this land connection. While Sicily was occupied as early as the Aurignacian, it may not have been until the Late Epigravettian that the island had a stable population able to overcome the threat of extinction. Like the rest of Italy, Sicily gives us a unique insight the challenges faced by Europe’s latest inhabitants.
*BP is used to indicated uncalibrated radiometric years before present.
References
Bocquet-Appel et al. Estimates of Upper Palaeolithic meta-population size in Europe from archaeological data. Journal of Archaeological Science (2005) vol. 32 (11) pp. 1656-1668.
Chilardi S, Frayer DW, Gioia P, Macchiarelli R, Mussi M (1996) Fontana Nuova di Ragusa (Sicily, Italy): southernmost Aurignacian site in Europe 70: 553-563.
D'Amore G, Marco SD, Tartarelli G, Bigazzi R, Sineo L (2009) Late Pleistocene human evolution in Sicily: comparative morphometric analysis of Grotta di San Teodoro craniofacial remains 56: 537-550.
Ferembach. Les Hommes du Paléolithique Supérieur. Autour du Bassin Méditerraneen. L'Anthropologie (1986) vol. 90 (3) pp. 579-587.
Henke W (1991) Biological Distances in Late Pleistocene and Early Holocene Human Populations in Europe. In: Variability and Evolution. Poznan, Poland: Miskiewicz University Press. pp. 39-64.
Henke (1989) Jungpaläolithiker und Mesolithiker: Beiträge zur Anthropologie. Habilitationsschrift, FB Biologie, Mainz, 1701 S.
Holt BM (2003) Mobility in Upper Paleolithic and Mesolithic Europe: Evidence from the lower limb. Am. J. Phys. Anthropol.
Mussi (1990) Continuity and change in Italy at the Last Glacial Maximum.. In: Soffer, Gamble, editors. The world at 18000: high latitudes. London: . pp. 126-147.
Mussi M (2000) Heading south: the gravettian colonisation of Italy. In: Roebroeks, Mussi, Svoboda, Fennema, editors. Hunters of the Golden Age: The Mid Upper Palaeolithic of Eurasia 30,000–20,000 BP. Leiden: Leiden University Press. pp. 355-374.
Who made the Aurignacian?
Sat, May 30 2009 03:40 | Palaeoanthropology | Permalink
Until recently, it was largely assumed that the Aurignacian was contemporaneous with the arrival of anatomically modern humans in Europe sometime around 40,000 years ago. This industrial complex is named after the site of Aurignac in southern France and is found throughout Europe and southwest Asia. The evidence for an association between modern humans the Aurignacian has been less than clear cut.
For most of the last century, the prevalent view among archaeologists was that Neandertals only made Mousterian tools. However, the discovery of the St Césaire 1 Neandertal skeleton and the Neandertal remains from Arcy-sur-Cure with Châtelperronian industry put paid to this idea. Châtelperronian tools show a mix of features otherwise found in the Mousterian and Aurignacian industries. What was particularly surprising about the Châtelperronian culture was not only the lithics but also the manufacture of bone tools and personal ornaments. At the site of Arcy-sur-Cure archaeologists found pierced teeth, ivory, shell, and bone in the Châtelperronian layers. The Szeletian industry of central Europe and the Uluzzian industry of Italy may also be related to the Châtelperronian.
The Châtelperronian has shown that Neandertals were more skilful than previously thought and has opened up the possibility that they may have been the authors of the Aurignacian. At Vindija Cave in Croatia Neandertal remains were speculated to be associated with an Aurignacian-like assemblage (Smith et al. 1999). The skeletal fragments from this site show clear Neandertal affinities. However, problems with stratigraphic control during excavations, as well as evidence of cryoturbation and bioturbation mean that the Neandertal-Aurignacian association is questionable.
However, many of the best associations of the Aurignacian with modern humans are equally problematic. At Bacho Kiro Cave in Bulgaria a "proto-Aurignacian" culture has been associated with some fragmentary human remains (Kozłowski 1982). However, dates from the "proto-Aurignacian" layer span over thousands of years suggesting (1) a very long accumulation of sediment, (2) contamination or (3) incorrect context. Moreover, the fragmentary nature of the remains has meant that a taxonomic diagnosis is difficult.
Human remains were found at the Aurignacian levels of the Spanish site of El Castillo. Unfortunately, the remains were later lost before a detailed anatomical description could be published. A subsequent assessment by Garralda of available descriptions (1989) suggests that the remains were robust, a trait common to both Neandertals and early modern humans. Other sites such as Hahnöfersand and Vogelherd (Street et al. 2006), once thought to date to the Aurignacian have since been dramatically redated to more recent periods.
Evidence of a modern human-Aurignacian association are somewhat better at the sites of Zlatý kůň in the Czech Republic and Kent's Cavern in England. Perhaps, the best evidence we have comes from the site of Mladeç in the Czech Republic. Bone points, perforated animal teeth and a few lithics have been found there. The assemblage appears to be Aurignacian and is associated with skeletal remains that have been well dated to around 31,000 radiocarbon years.
A recent study by Bailey et al. (2009) attempts to shed further light on the makers of the Aurignacian. Many Aurignacian sites have dental remains but they largely have not been used in taxonomic identification. The authors of this paper use Bayesian statistics to classify individual based on the teeth. They used teeth samples for which taxonomy was known to test the accuracy of their technique. In cross validation of known samples, 89% of both Neandertals and modern humans were correctly classified. In the subsequent analysis of the 34 unknown samples associated with Upper Palaeolithic industries, 29 were assigned to modern humans. This is perhaps the strongest evidence to date that modern humans made the Aurignacian. However, this study cannot completely rule out the possibility that Neandertals could have been responsible, albeit for in small part, for the Aurignacian.
References
Bailey SE, Weaver TD, Hublin J-J. 2009. Who made the Aurignacian and other Upper Paleolithic industries? Journal of Human Evolution.
Garralda MD. 1989. Upper Paleolithic human remains from El Castillo Cave (Santander, Spain). In: Giacobini G, editor. Hominidae: Proceedings of the 2nd International Congress of Human Paleontology. Turin: Jaca Book. pp. 479-482.
Kozłowski JK. 1982. Excavation in the Bacho Kiro Cave (Bulgaria): final report. Warsaw: Panstwowe Wydawnictwo Naukowe.
Smith FH, Trinkaus E, Pettitt PB, Karavanić I, Paunović M. 1999. Direct radiocarbon dates for Vindija G1 and Velika Pećina late Pleistocene hominid remains. Proc Natl Acad Sci USA 96.
Street M, Terberger T, Orschiedt J. 2006. A critical review of the German Paleolithic hominin record. Journal of Human Evolution 51(6).

Above photo by Wessex Archaeology under creative commons license.
For most of the last century, the prevalent view among archaeologists was that Neandertals only made Mousterian tools. However, the discovery of the St Césaire 1 Neandertal skeleton and the Neandertal remains from Arcy-sur-Cure with Châtelperronian industry put paid to this idea. Châtelperronian tools show a mix of features otherwise found in the Mousterian and Aurignacian industries. What was particularly surprising about the Châtelperronian culture was not only the lithics but also the manufacture of bone tools and personal ornaments. At the site of Arcy-sur-Cure archaeologists found pierced teeth, ivory, shell, and bone in the Châtelperronian layers. The Szeletian industry of central Europe and the Uluzzian industry of Italy may also be related to the Châtelperronian.
The Châtelperronian has shown that Neandertals were more skilful than previously thought and has opened up the possibility that they may have been the authors of the Aurignacian. At Vindija Cave in Croatia Neandertal remains were speculated to be associated with an Aurignacian-like assemblage (Smith et al. 1999). The skeletal fragments from this site show clear Neandertal affinities. However, problems with stratigraphic control during excavations, as well as evidence of cryoturbation and bioturbation mean that the Neandertal-Aurignacian association is questionable.
However, many of the best associations of the Aurignacian with modern humans are equally problematic. At Bacho Kiro Cave in Bulgaria a "proto-Aurignacian" culture has been associated with some fragmentary human remains (Kozłowski 1982). However, dates from the "proto-Aurignacian" layer span over thousands of years suggesting (1) a very long accumulation of sediment, (2) contamination or (3) incorrect context. Moreover, the fragmentary nature of the remains has meant that a taxonomic diagnosis is difficult.
Human remains were found at the Aurignacian levels of the Spanish site of El Castillo. Unfortunately, the remains were later lost before a detailed anatomical description could be published. A subsequent assessment by Garralda of available descriptions (1989) suggests that the remains were robust, a trait common to both Neandertals and early modern humans. Other sites such as Hahnöfersand and Vogelherd (Street et al. 2006), once thought to date to the Aurignacian have since been dramatically redated to more recent periods.
Evidence of a modern human-Aurignacian association are somewhat better at the sites of Zlatý kůň in the Czech Republic and Kent's Cavern in England. Perhaps, the best evidence we have comes from the site of Mladeç in the Czech Republic. Bone points, perforated animal teeth and a few lithics have been found there. The assemblage appears to be Aurignacian and is associated with skeletal remains that have been well dated to around 31,000 radiocarbon years.
A recent study by Bailey et al. (2009) attempts to shed further light on the makers of the Aurignacian. Many Aurignacian sites have dental remains but they largely have not been used in taxonomic identification. The authors of this paper use Bayesian statistics to classify individual based on the teeth. They used teeth samples for which taxonomy was known to test the accuracy of their technique. In cross validation of known samples, 89% of both Neandertals and modern humans were correctly classified. In the subsequent analysis of the 34 unknown samples associated with Upper Palaeolithic industries, 29 were assigned to modern humans. This is perhaps the strongest evidence to date that modern humans made the Aurignacian. However, this study cannot completely rule out the possibility that Neandertals could have been responsible, albeit for in small part, for the Aurignacian.
References
Bailey SE, Weaver TD, Hublin J-J. 2009. Who made the Aurignacian and other Upper Paleolithic industries? Journal of Human Evolution.
Garralda MD. 1989. Upper Paleolithic human remains from El Castillo Cave (Santander, Spain). In: Giacobini G, editor. Hominidae: Proceedings of the 2nd International Congress of Human Paleontology. Turin: Jaca Book. pp. 479-482.
Kozłowski JK. 1982. Excavation in the Bacho Kiro Cave (Bulgaria): final report. Warsaw: Panstwowe Wydawnictwo Naukowe.
Smith FH, Trinkaus E, Pettitt PB, Karavanić I, Paunović M. 1999. Direct radiocarbon dates for Vindija G1 and Velika Pećina late Pleistocene hominid remains. Proc Natl Acad Sci USA 96.
Street M, Terberger T, Orschiedt J. 2006. A critical review of the German Paleolithic hominin record. Journal of Human Evolution 51(6).
Above photo by Wessex Archaeology under creative commons license.
A little human with very big feet
Wed, May 13 2009 02:50 | Palaeoanthropology | Permalink

The pelvis and legs all clearly demonstrate that Homo floresiensis was bipedal. However, the hominin’s feet are unusually long compared to the leg. This combination of a long foot and a relatively short leg is seen in some apes but not in hominins. The navicular acts like the keystone in the arch of the human foot and is elevated from the ground except in people who have fallen arches. In Homo floresiensis this bone has a well developed tuberosity meaning that it was in contact with the ground, like in other flat-footed great apes and early hominins. The overall shape of the foot means that this hominin would not have been able to run long distances very efficiently — a distinguishing feature of later Homo. While the hominin had a short chimp-like big toe, it was not opposable like in other hominins. The long, curved lateral toes resemble a chimpanzee’s, rather than those of a human which are short and straight. The distal first metatarsal is squared off like in modern humans but this feature is not found in other early hominins, such as Australopithecus afarensis, Paranthropus robustus or the early Homo remains from the Georgian site of Dmanisi.
It is generally thought that Homo erectus was the first hominin to leave Africa, soon after their first appearance in the archaeological record around 1.9 million years ago. Based solely on archaeological data Homo erectus seems like the best ancestor for Homo floresiensis. Homo erectus has been show to be a highly variable species. Homo floresiensis could represent a descendent of Homo erectus that adapted to island life through a dramatic decrease in size. While some of the plesiomorphic traits of Homo floresiensis may be explained through evolutionary reversals, it is unlikely to account for all of the primitive traits in the skeleton as a whole. It has been suggested that the ancestor of Homo floresiensis was not Homo erectus but rather a more primitive hominin. The 1.8 million year old skeletal Homo remains from Dmanisi are relatively primitive. At first glance, this species might seem like a good ancestral candidate for Homo floresiensis. However, unlike Homo floresiensis, the Dmanisi specimens have quite modern limb proportions. Homo habilis has also been forwarded as a possible candidate although there is little archaeological evidence to suggest that that species ever left Africa. Until further evidence comes along, the jury is out on this miniature human.
Reference cited
Jungers et al. The foot of Homo floresiensis. Nature 459, 81-84 (7 May 2009) | doi:10.1038/nature07989
Above photo modified from original by Mamoritai under creative commons license.